N 2 g nitrogen 3H 2 g hydrogen heat pressure catalyst 2NH 3 g. There has been recent progress which goes beyond incremental process technology improvements in ammonia production to yield disruptive and even breakthrough advancements.
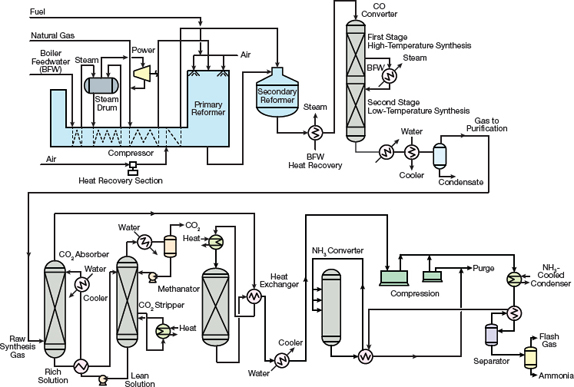 Introduction To Ammonia Production Aiche
Introduction To Ammonia Production Aiche
A flow scheme for the Haber Process looks like this.

Manufacture of ammonia by haber process pdf. 9 1913 with a production capacity of 30 mtday. This process produces an ammonia NH 3 g yield of approximately 10-20. Current practice to obtain N 2 employs.
2020-08-15 The Haber Process is used in the manufacturing of ammonia from nitrogen and hydrogen and then goes on to explain the reasons for the conditions used in the process. 2019-04-24 The source of this ammonia was the Haber-Bosch process and though some say its one of the most significant achievements of all time it comes with a heavy price. This and the next few spreads look at the Haber process in detail.
2020-11-24 Ammonia produced via the Haber-Bosch HB process is globally the leading chemical in energy consumption and carbon dioxide emissions. The plant went on-stream on Sept. In ammonia plants hydrogen is generated by steam-methane.
In the Haber Process ammonia NH3 is synthesised from nitrogen and hydrogen gases. What shall we do so many conflicting factors. The reaction is reversible and the production of ammonia is exothermic.
Some notes on the conditions. The Haber-Bosch process only converts 10 percent of its source material per cycle so needs to run multiple times to use it all up. This energy-intensive process.
And production methods for large-scale production of ammonia. Manufacture of ammonia by the Haber Process. The reaction is reversible and the production of ammonia is exothermic.
CO 2 emissions are at least 2x the production volume. The manufacturing of ammonia Nitrogen from air and hydrogen from natural gas methane - C H 4 or the cracking of hydrocarbons are reacted to make ammonia. The Haber synthesis was developed into an industrial process by Carl Bosch.
The leading method for the industrial production of ammonia has been the Haber-Bosch process for nearly a century worldwide. According to Le Chateliers Principle. The reactor contained an internal heat exchanger in addition to those shown on the schematic.
The haber process Ammonia is produced using the Haber Process At the plant hydrogen and nitrogen are mixed in the ration 31 by volume The gas pressure is raised to 200 atm atmospheres. A brief summary of the Haber Process. Figure 1 is a flowsheet of the first commercial ammonia plant.
Almost all the ammonia used throughout the world is manufactured by the process developed by Fritz Haber at the beginning of the twentieth century. Today this process produces 500 million tons of nitrogen fertilizer per year and is responsible for sustaining one-third of the Earths population. Catalyst and related process.
This process is known for extremely high pressures that are required to maintain a reasonable equilibrium constant. We shall reach a compromise. The first step in making nitrogen fertiliser is to make ammonia from the nitrogen in the air and hydrogen.
The reaction between nitrogen gas and hydrogen gas to produce ammonia gas is exothermic releasing 924kJmol of energy at 298K 25oC. Energy consumption by ammonia production is the largest in the chemical industry. The collaborative efforts of Haber andBosch made the commercial high-pressure synthesis of ammonia possible by 1913.
2002-09-01 N 2 is an industrial gas with a wide array of chemical and medical applications including in the production of ammonia via the Haber-Bosch process 1. A lower temperature also. The process combines nitrogen from the air with hydrogen derived mainly from natural gas methane into ammonia.
The overall process requires high temperatures and pressures and utilizes nitrogen fixation reacting atmospheric nitrogen continuous flow and the frequent recovery of unreacted gases resulting in a method capable of producing large amounts of ammonia. The first commercial ammonia plant based on the Haber-Bosch process was built by BASF at Oppau Germany. A high pressure and low temperature will produce optimum yield 600 x102kPa 600 atm and 473K 200oC produce near 100 yield.
The first commer-cial plant with a capacity of 30 tonsday was set up by the German chemical giant BASF Badashe Analine und Soda Fabrik in Oppau Germany 2. The Haber Process combines nitrogen from the air with hydrogen derived mainly from natural gas methane into ammonia. Fritz Haber and Carl Bosch developed a process for the manufacture of ammonia on an industrial scale Haber-Bosch process.
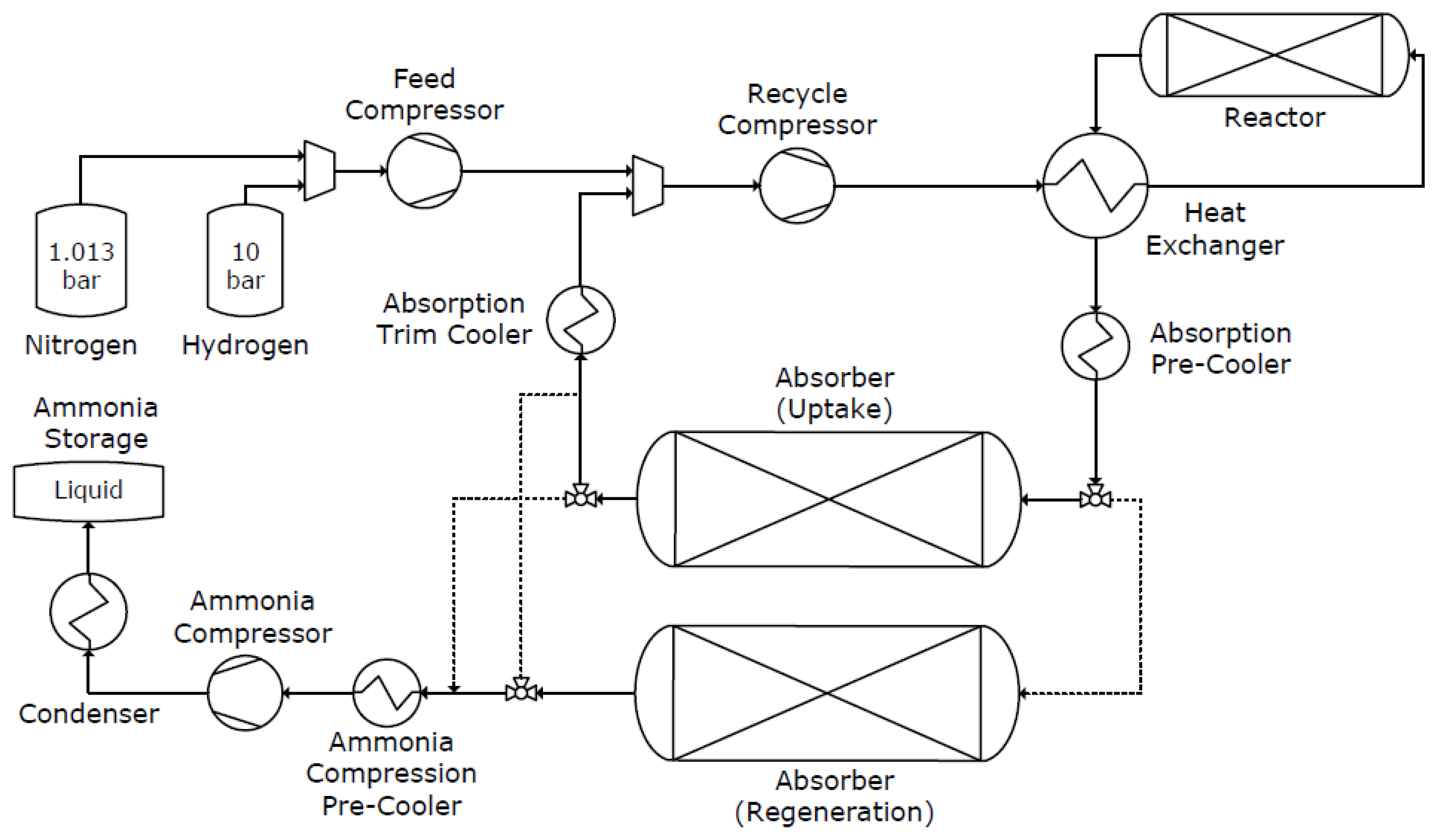 Processes Free Full Text Modeling And Optimal Design Of Absorbent Enhanced Ammonia Synthesis Html
Processes Free Full Text Modeling And Optimal Design Of Absorbent Enhanced Ammonia Synthesis Html
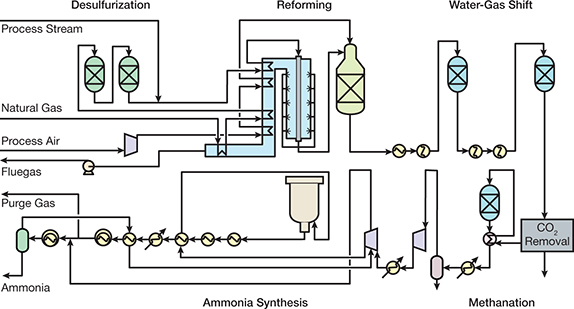 Introduction To Ammonia Production Aiche
Introduction To Ammonia Production Aiche
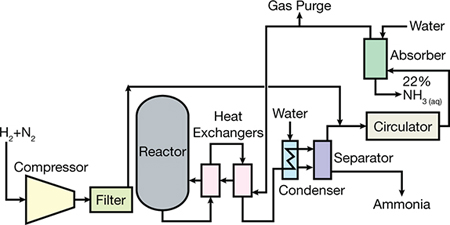 Introduction To Ammonia Production Aiche
Introduction To Ammonia Production Aiche
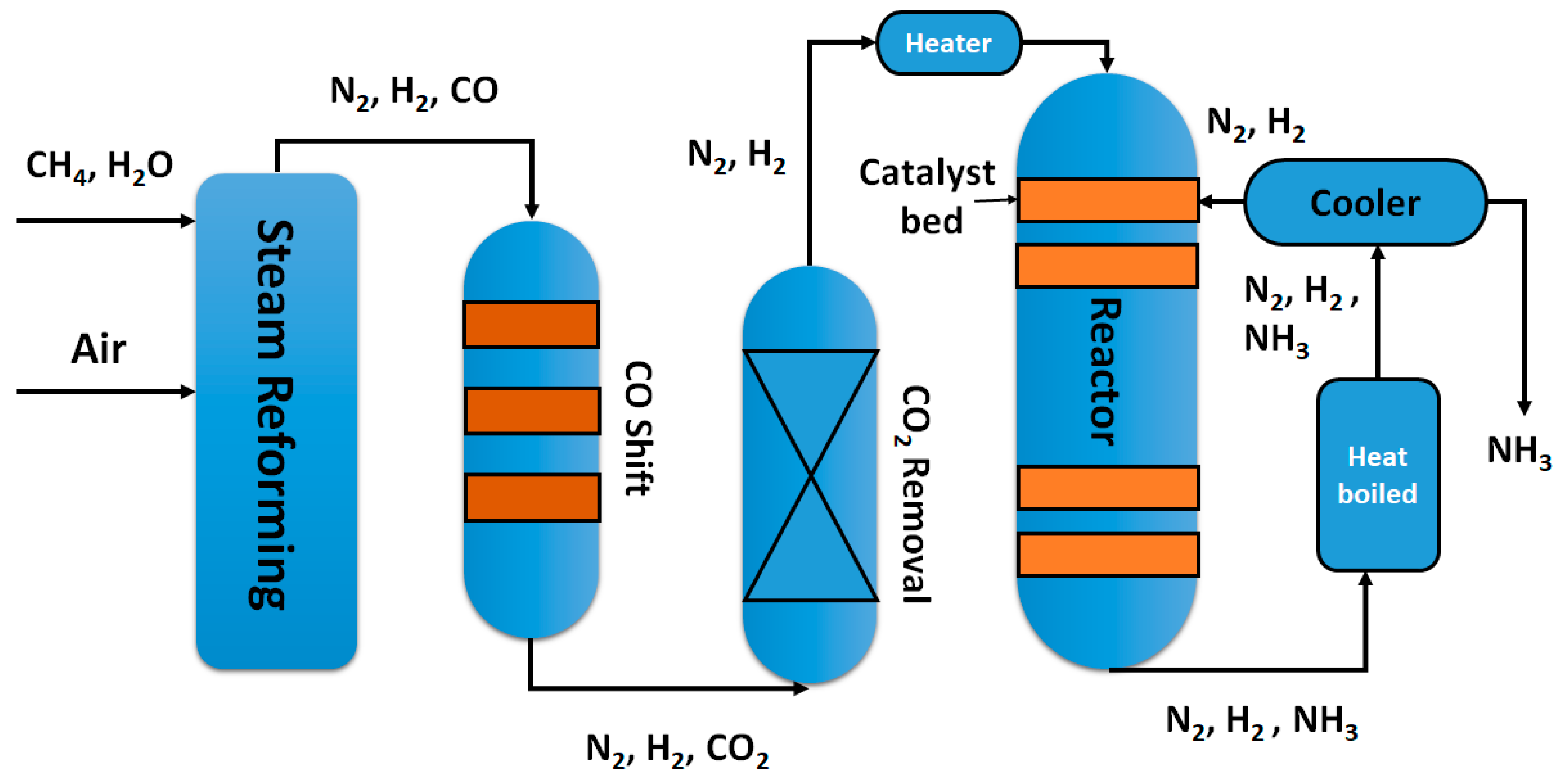 Catalysts Free Full Text Insights Into The Recent Progress And Advanced Materials For Photocatalytic Nitrogen Fixation For Ammonia Nh3 Production Html
Catalysts Free Full Text Insights Into The Recent Progress And Advanced Materials For Photocatalytic Nitrogen Fixation For Ammonia Nh3 Production Html
